
G-proteins can be found on the inner surface of cell membranes. They consist of three subunits, a , b , and g . In the resting state all three subunits are anchored to the membrane through a fatty acid chain, attached to an amino acid residue through a reaction known as prenylation. However, G-proteins are free to diffuse in the plane of the membrane.
There exist three different families of G-proteins: Gs, Gi, and Gq/11, which show selectivity with respect to receptors and to effectors. Gs and Gi produce stimulation and inhibition of the enzyme adenylate cyclase respectively, whilst Gq/11 interacts with phospholipase C. The a -subunits of these G-proteins differ in structure
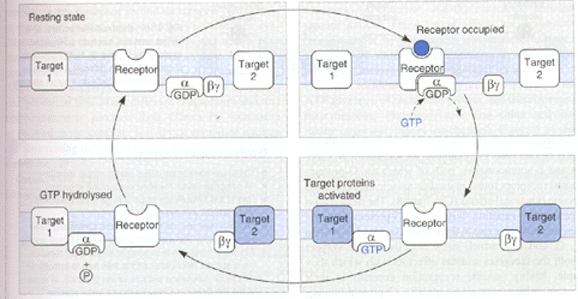
Diagram from Rang, Dale, and Ritter, "Pharmacology" Fourth Edition,1999
When a drug molecule binds to a G-protein linked receptor it causes a change in the conformation of the receptor. The G-protein recognises this change in the receptor and binds to it. The receptor triggers the exchange of bound GDP for GTP on the a subunit of the G-protein. The a -GTP (active form) moves away from the b and g subunits as a -GTP no longer fits comfortably with the b and g units.
The a -GTP associates with various enzymes and ion channels, causing activation or inactivation. The a -GTP becomes hydrolysed to a -GDP through the GTPase activity of the a -subunit. Both the b and g subunits are attracted to the a subunit in its a -GDP form. As a consequence a -GDP joins back with the b and g units. G-proteins therefore show a cyclic behaviour.
The a -subunits GTPase activity increases once it becomes bound to an effector molecule. The degree of increase in a -GTPase activity is dependent upon the effector molecule. Since GTP hydrolysis is the terminating step of the process, regulation of the a -GTPase activity by the effector protein means that the activation of the effector is essentially self limiting.
Studies using site directed mutagenesis have shown that the receptor and G-protein interact through the third intracellular loop. The third intracellular loop in adrenoceptors represents the third site of greatest diversity between these classes. The character of each loop would appear to determine which G-protein the receptor is preferentially coupled to. The adrenoceptor classes work through different G-proteins.
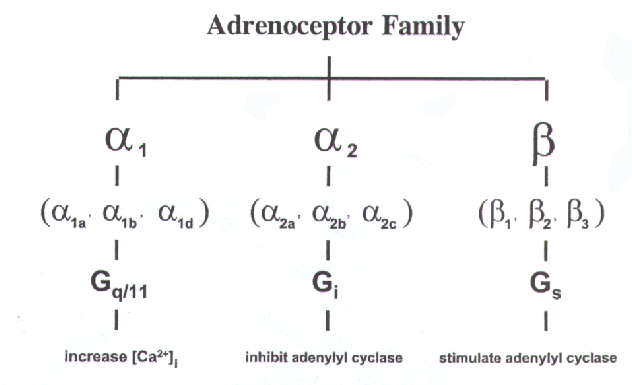
Diagram from H.Zhong, K.P. Minneman/European Journal of Pharmacology 375 (1999) 261-276
Other portions of the adrenoceptor molecule contribute to the interactions with G-proteins. Studies with the a2-adrenoceptor suggest that mutation at the Asp79 site interferes with G-protein dependent agonist-receptor interaction. Also Asp113 has been shown to have an effect on agonist and antagonist binding to the adrenoceptors, being implicated in the conformational changes that take place in the receptor upon agonist binding.
So long as the agonist is bound to the receptor, G-proteins will continue to bind to the receptors changed conformation and so many molecules of effector will become activated. The process stops when the drug leaves the receptor and the receptor goes back to its normal conformation. The size of the response in this system is therefore due to the affinity of the agonist to the receptor, and tends to result in amplification of the initial response.
.