
The b3 adrenoceptor has a profile quite distinct from that of b1 and b2 adrenergic receptors. Rodents, humans and other mammals share many of the characteristic b3 properties, although observable species-species differences have been identified. The naturally occurring variant of the human b3 receptor was correlated with hereditary obesity in Pima Indians, in Japanese individuals and in Western obese patients. Weight gain was also observed in female mice whose b3 gene had been disrupted.
These examples provided a picture of the important role of the b3 receptor in the regulation of lipid metabolism and as a possible target for drugs to treat certain forms of obesity.
Early antagonist/agonist studies of b adrenergic receptors suggested the existence of atypical responses clearly different from effects mediated by the b1 and b2 adrenoceptors. The new agonist BR37344 stimulated lipolysis in rodent brown adipocytes even in the presence of the antagonist propranolol. This and other discoveries led to the notion that additional receptors existed in fat, in the gut, in the heart and possibly in skeletal muscle. Cloning, sequencing and expression of the b3 gene in various species has now served to confirm the existence of this receptor.
The b3 adrenoceptor is composed of a single 408 amino acid residue peptide chain that belongs to the family of G-protein coupled receptors. The G-protein receptors are characterised by seven hydrophobic stretches of about 22 to 28 residues forming seven transmembrane segments. The transmembrane (TM) regions are linked with three intracellular and three extracellular loops. The amino acid (N) terminal of these receptors is located extacellularly and is glycosylated. The carboxy (C) terminal is intracellular and the case of the b3 receptors does not have any phosphorylation sites.
Comparison of the b receptor subtypes revealed that there were a limited number of conserved residues and these were mainly restricted to the transmembrane segments and to the proximal regions of the intracellular loops.
Comparing b3 with b3 receptors of other species revealed a high degree of sequence homology approximately 80-90% between human, bovine, rodent and canine. The human, monkey and bovine b3 receptors are closer to each other than any of the rodents sequences, in particular in transmembrane segment one. Closer inspection of the various b3 sequences revealed that the human b3 is distinct from is animal relatives in several positions occupied by one type of residue in humans and another type in animals. One particular example of this occurs in position 64, which is occupied in humans by a tryptophan residue and an arginine in all other species.
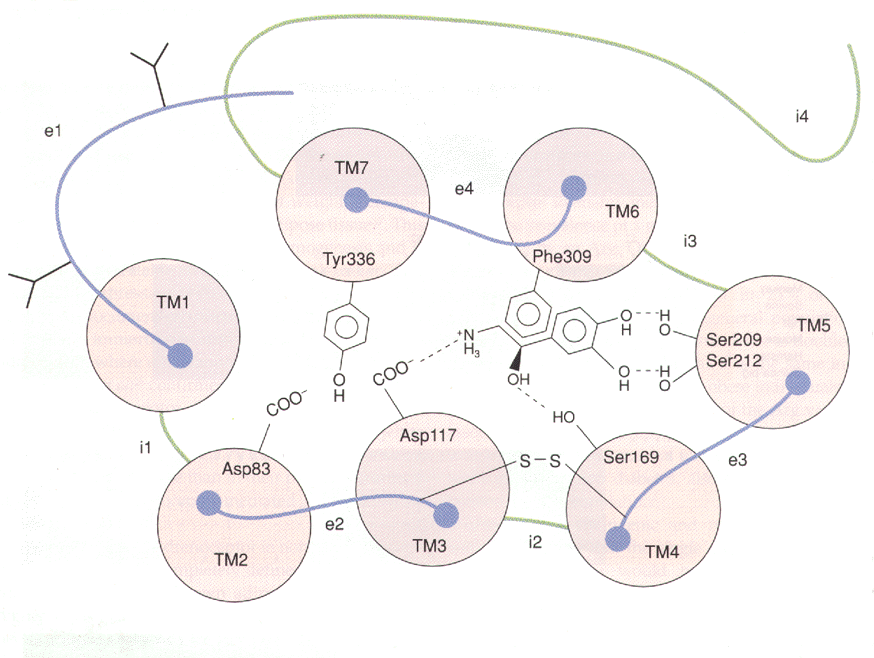
Computer modelling has defined an image of the b3 ligand-binding site. At least four of the seven transmembrane domains are essential for ligand binding (see diagram below). The amino acids that are involved were identified by site-directed mutagenesis and photoaffinty labelling these are Asp117 in TM3, a residue found to be essential for binding all biogenic amines. Ser169 in TM4, is thought to form a hydrogen bonds with the hydroxyl of the ethanolamine side-chain. Ser209 and Ser212 in TM5, also located in many biogenic amine receptors, are thought to form hydrogen bonds with the hydroxyls of the catechol side chain. Also Phe309 in TM6, involved in hydrophobic interactions with the aromatic ring of catecholamines. Two of the three TM domains are involved in Gs activation, TM2, which contain Asp83, and TM7, which contains Tyr336.
Attempts to explain why several b2 antagonists behave as b3 agonist have not yet been successful. b3 receptors appear to contain less bulky residues, and could therefore accommodate the larger b1/b2 antagonists. Mutagenesis has been used to substitute the small Gly residue with the larger Phe residue at position 53 in the b3 receptor; this change however was not enough to convert the b3 agonist to an antagonist.
The Gs interaction site on b3 is situated in the intracellular region, mainly the membrane proximal regions of the second and third (i2, i3) intracellular loops and the carboxy-terminal domains. Deletion of small segments of the amino terminal and carboxy terminal regions of the i3 of the b3 receptor uncoupled the human receptor from adenylate cyclase upon agonist stimulation.
Several reports propose that the b3 receptor may be coupled to more than one second messenger system. It has been shown that b3 receptors in rat adipocytes interact with both Gs and Gi proteins. The b3 adrenergic receptor in the septum of the human heart has also been reported to link with the Gi protein, resulting in a negative inotropic effect. This is in contrast to previous observations that always associated b receptors with positive inotropism in the heart.
b3 adrenoceptors are primarily expressed in brown and white adipose tissue (BAT and WAT), where it is thought to regulate noradrenaline-induced changes in energy metabolism and thermogenesis. Several reports have also indicated the existence of b3 receptors in additional locations, where the receptor may modulate other functions. As is the case of the gastro-intestinal tract where b3 may modulate intestinal motility by causing vaso-relaxation or protect the mucosal surface. Expression of b3-like properties in cardiac tissue has also been well documented.
The evidence in support of the b3 adrenergic receptor modulating energy metabolism and thermogenesis comes from studies on rodents where b3 agonists considerably reduced diet-induced obesity. Evidence was also collected from experiments carried out in adult dogs treated with b3 agonist ICI D7114 for two month, the dogs showed a reduction in weight and also showed an increase in BAT (the tissue mostly responsible for thermogenesis normally undetected in adult mammals other than rodents).
The role of the b3 receptor in adipocytes is now well-documented in rodents, where it is the main subtype in white and brown adipocytes. In these cells the b3 is coupled to adenylate cyclase III, stimulation of which generates cAMP that phosphorylates the hormone sensitive protein lipoprotein lipase responsible for lipolysis of triglycerides. In white adipocytes, the resulting free fatty acids are either released in serum or reassembled in triglycerides. In brown adipocytes the free fatty acids are oxidised into CO2 and water releasing energy in the form of heat.
Due this role in fat metabolism in rodents and its predominant expression in human fat tissues the b3 receptor in now widely considered a valuable pharmacological target for the treatment of obesity. The development of a selective b3 agonist therefore constitutes the most realistic approach to a useful agent for the treatment of human obesity. Such a compound, rather than support an effort to inhibit appetite, would help reduce accumulated fat rendering this approach more attractive.